Own Values (ILSB Method)
The values given here are determined with electric potential difference measurements by our group. They are self-consistent and in accordance with TATB data. We consider the data to be the most reliable currently available. Note that the work is still in progress.
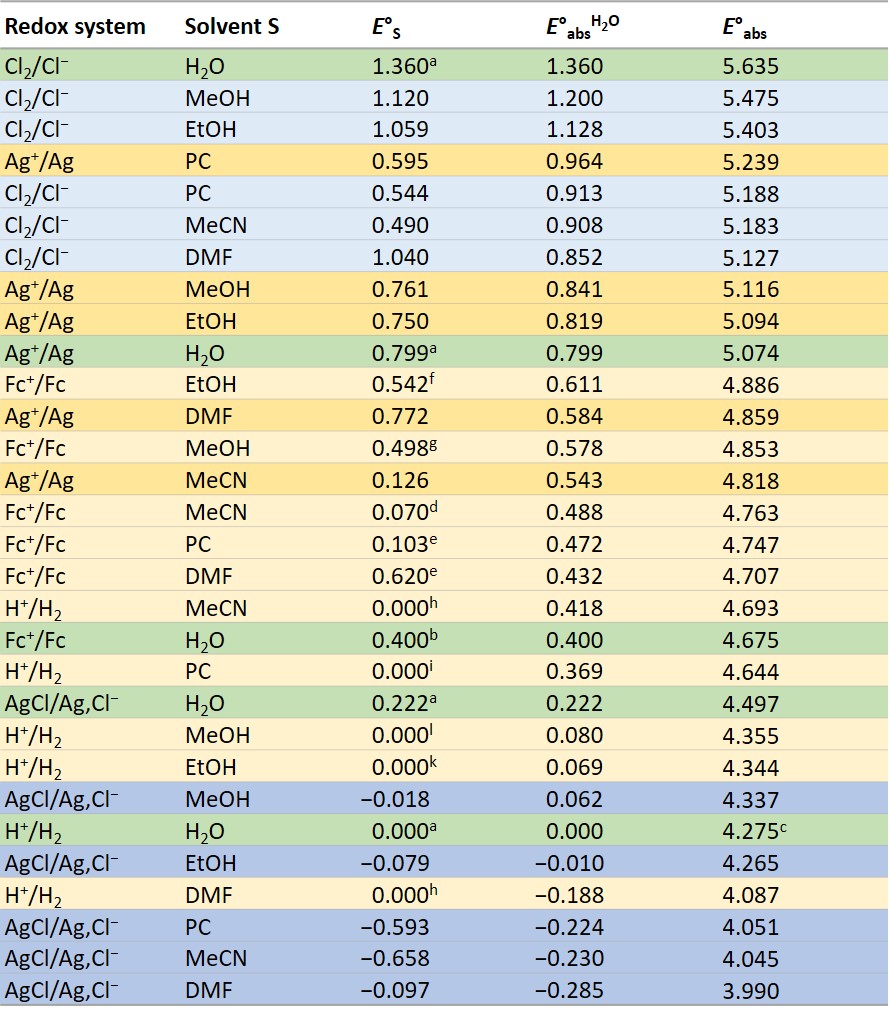
All values are given in V. The green shaded values are reference values, the yellow shaded values werde determined with cell I, and the blue shaded values with cell II, the light blue shaded values can be derived from them. The light yellow values were obtained with values from cell I and values of measurements in the solvent S between the redox systems Ag+/Ag vs H+/H2 or Ag+/Ag vs Fc+/Fc or additionally Fc+/Fc vs H+/H2 from literature (see below).
Ag│AgCi (c, S2) │ [N2225][NTf2] │ AgCi (yc, S1)│Ag cell I
Ag│AgCl│CiCl (c, S2)│[N2225][NTf2]│CiCl (yc, S1)│AgCl│Ag cell II
Ci is an appropriate counterion. [N2225][NTf2] is the pure ionic liquid used in the salt bridge. For details see: 1
A. Ermantraut, V. Radtke, N. Gebel, D. Himmel, T. Koslowski, I. Leito, I. Krossing, Angew. Chem. Int. Ed. 2018, 57, 2348.
V. Radtke, N. Gebel, D. Priester, A. Ermantraut, M. Bäuerle, D. Himmel, R. Stroh, T. Koslowski, I. Leito, I. Krossing, Chem. Eur. J. 2022, e202200509.
V. Radtke, D. Priester, A. Heering, C. Müller, T. Koslowski, I. Leito, I. Krossing, Chem. Eur. J. 2023, 29, e202300609.
a) S. G. Bratsch, J. Phys. Chem. Ref. Data 1989, 18, 1
b) A. M. Bond, E. A. McLennan, R. S. Stojanovic, F. G. Thomas, Anal. Chem. 1987, 59, 2853
c) M. D. Tissandier, K. A. Cowen, W. Y. Feng, E. Gundlach, M. H. Cohen, A. D. Earhart, J. V. Coe, T. R. Tuttle, J. Phys. Chem. A 1998, 102, 7787
d) I. M. Kolthoff, F. G. Thomas, J. Phys. Chem. 1965, 69, 3049
e) A. V. Benedetti, Eclet. Quim. J. 1984, 9, 13
f) J. W. Diggle, A. J. Parker, Electrochim. Acta 1973, 18, 975
g) R. Alexander, A. J. Parker, J. H. Sharp, W. E. Waghorne, J. Am. Chem. Soc.1972, 94, 1148
h) V. Fourmond, P.-A. Jacques, M. Fontecave, V. Artero, Inorg. Chem. 2010, 49, 10338
i) L. M. Mukherjee, R. G. Bates, J. Electroanal. Chem. Interfacial Electrochem.1985, 187, 73
k) A. Macfarlane, H. Hartley, Philos. Mag. 1932, 13, 425.
l) P. S. Buckley, H. Hartley, Philos. Mag. 1929, 8, 320
